Introduction
Acetic acid (CH₃COOH) is a vital compound in organic chemistry, commonly known as the key ingredient in vinegar. It plays an essential role in industrial applications, biochemistry, and laboratory research. Understanding its formula, structure, and reactions is crucial for students preparing for chemistry exams and researchers working on sustainable production methods.
This article explores the molecular formula, structure, physical and chemical properties, production methods, and diverse applications of acetic acid. Whether you’re a student revising for an exam or a researcher exploring its industrial uses, this guide provides valuable insights.
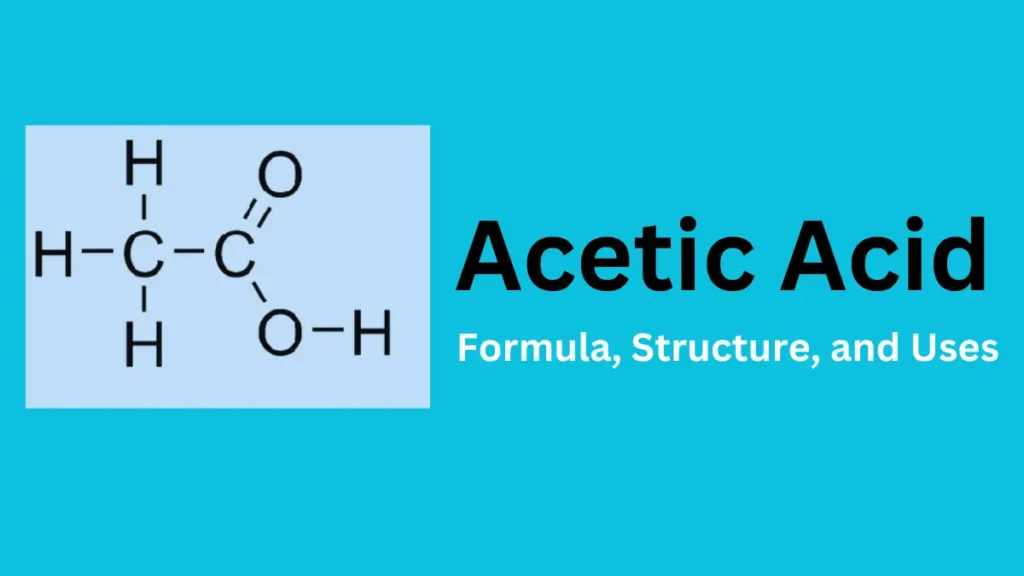
Chemical Formula and Molecular Structure
Empirical and Molecular Formula
- Molecular Formula: C₂H₄O₂
- Structural Formula: CH₃COOH (showing the presence of a carboxyl group -COOH).
Structural and Bonding Features
- Molecular Geometry: Planar structure with sp² hybridization in the carboxyl group.
- Hydrogen Bonding: Strong intermolecular hydrogen bonds contribute to its relatively high boiling point (118°C).
- Spectroscopic Data:
- Infrared (IR) Spectrum: O-H stretch (~3000 cm⁻¹), C=O stretch (~1700 cm⁻¹).
- NMR Data: Methyl protons at δ 2.1 ppm, carboxyl proton at δ 11-12 ppm.
Physical and Chemical Properties
Physical Properties
- Appearance: Colorless liquid with a sharp, pungent odor.
- Melting Point: 16.6°C (pure acetic acid freezes to form ice-like crystals, hence the term “glacial acetic acid”).
- Solubility: Fully miscible in water, ethanol, and ether.
Chemical Properties
- Acidity: Weak acid (pKa = 4.76), but stronger than most carboxylic acids due to resonance stabilization.
- Key Reactions:
- Esterification: Reacts with ethanol to form ethyl acetate, an important solvent.
- Neutralization: Forms acetate salts when reacting with bases (e.g., sodium acetate).
- Reduction: Can be reduced to acetaldehyde or ethanol using appropriate reducing agents.
Production Methods
1. Fermentation (Traditional Method)
- Used in vinegar production, where ethanol is oxidized by Acetobacter bacteria.
- Reaction: C₂H₅OH + O₂ → CH₃COOH + H₂O
2. Modern Industrial Methods
- Monsanto Process: Methanol carbonylation using rhodium catalysts, with over 90% efficiency.
- Cativa Process: Similar to Monsanto but uses an iridium catalyst for lower energy consumption.
- Bio-Based Production: Utilizes genetically modified bacteria for sustainable acetic acid production.
Applications and Uses
1. Food Industry
- Vinegar Production: Used as a preservative and flavor enhancer (4-8% acetic acid in water).
- Food Additive (E260): Acts as a pH regulator in food processing.
2. Industrial Applications
- Vinyl Acetate Monomer (VAM): Key raw material for adhesives, paints, and coatings.
- Plastic Production: Used in manufacturing PET plastic and synthetic fibers.
- Solvent: Plays a role in terephthalic acid production for polyester fibers.
3. Pharmaceutical and Medical Uses
- Antiseptic Agent: Diluted acetic acid solutions are used for wound care.
- Drug Synthesis: An intermediate in the production of aspirin and antibiotics.
4. Laboratory Applications
- Buffer Solutions: Acetate buffers maintain pH stability in biochemical reactions.
- Organic Synthesis: Used in acetylation reactions to modify organic molecules.
Safety and Handling
- Hazards: Corrosive to skin and eyes, can cause severe burns.
- Protective Measures: Use gloves, goggles, and work in a well-ventilated area.
- Disposal: Neutralize with sodium bicarbonate before disposal to prevent environmental harm.
Conclusion
Acetic acid is a fundamental compound in organic chemistry with widespread academic and industrial significance.
Key Takeaways for Students:
- Exam Preparation: Learn its structural formula, acidity, and key reactions.
- Research Applications: Explore its role in sustainable production and industrial synthesis.
- Lab Safety: Follow proper safety protocols when handling concentrated solutions.
Exam Tip: Practice writing balanced chemical equations for esterification and neutralization reactions—these frequently appear in chemistry exams.
FAQs
1. Why is pure acetic acid called “glacial”?
It freezes at 16.6°C, forming ice-like crystals, hence the name “glacial acetic acid.”
2. How is vinegar different from pure acetic acid?
Vinegar contains only 4-8% acetic acid, along with water and other compounds, while pure acetic acid is undiluted.
3. What role does acetic acid play in metabolism?
It is a key component of acetyl-CoA, which is essential in the Krebs cycle for energy production in cells.
References
- Haynes, W. M. (Ed.). (2016). CRC Handbook of Chemistry and Physics. CRC Press.
- Smith, J. G. (2020). Organic Chemistry. McGraw-Hill Education.
- PubChem: Acetic Acid
- ACS Sustainable Chemistry: Bio-Based Production
Internal Links
- Vinegar Formula, Structure, and Properties – Complete Guide
- Vinegar Formula Explained: Chemical Structure, Uses, and Key Properties (CH₃COOH)
This guide ensures a deep understanding of acetic acid, making it a valuable resource for students, researchers, and industry professionals.
As a finance news writer at sirfal.com, I specialize in breaking down complex economic trends, market updates, and investment strategies into clear, actionable insights. My mission is to empower readers with the knowledge needed to make informed financial decisions. Thank you for engaging with my articles; I hope they add value to your financial journey.